Getting The Cannabinol To Work
Table of ContentsSome Known Details About Cannabinol The 10-Second Trick For CannabinolAbout CannabinolMore About CannabinolThe Facts About Cannabinol RevealedThe smart Trick of Cannabinol That Nobody is Discussing

CBD was not an ingredient considered under the OTC medicine evaluation. An unapproved new medication can not be distributed or marketed in interstate commerce. FDA remains to be worried at the expansion of items insisting to include CBD that are marketed for therapeutic or clinical usages although they have not been authorized by FDA.
Marketing unauthorized products with unverified restorative claims is not only an offense of the regulation, however additionally can place people in danger, as these products have actually not been confirmed to be safe or efficient. This misleading advertising of unverified therapies additionally raises substantial public health problems, due to the fact that people as well as various other consumers might be influenced not to use approved treatments to deal with significant and also also fatal conditions.
How Cannabinol can Save You Time, Stress, and Money.
The firm has, however, approved one cannabis-derived and also three cannabis-related medicine items (see Inquiry # 2). FDA counts on candidates as well as clinical investigators to carry out research. The firm's duty, as outlined in the FD&C Act, is to assess data submitted to the FDA in an application for authorization to make sure that the medication product satisfies the legal requirements for authorization.
Added information worrying research study on the clinical usage of cannabis is offered from the National Institutes of Wellness, especially the National Cancer Institute (NCI) and National Institute on Substance Abuse (NIDA). A. The FDA knows that numerous states have either passed laws that remove state restrictions on the clinical use of marijuana as well as its derivatives or are thinking about doing so.
We invite the chance to speak with states that are thinking about support for medical study of cannabis and its derivatives, to make sure that we can supply details on Federal as well as clinical criteria. A. The agency has actually received reports of adverse events in individuals making use of marijuana or cannabis-derived items to deal with medical problems.
Some Known Facts About Cannabinol.
Additional information regarding the safety as well as efficiency of marijuana and its components is required. Scientific tests of marijuana conducted under an IND application might accumulate this crucial details as a component of the drug growth process. A. cannabinol. It depends, to name a few points, on the planned use the product as well as how it is classified and marketed.
The listed below concerns and also responses discuss a few of the manner ins which particular parts of the FD&C Act can affect the legitimacy read the full info here of CBD products. We understand that state as well as regional authorities are fielding numerous concerns concerning the legality of CBD. There is ongoing interaction with state as well as local authorities to address questions about demands under the FD&C Act, to much better comprehend the landscape at the state degree, as well as to otherwise involve with state/local regulative partners.
FDA takes into consideration a compound to be "authorized for investigation as a brand-new medicine" if it is the subject of an Investigational New Medicine application (IND) that has actually entered into result. Under FDA's regulations (21 CFR 312. 2), unless a professional investigation meets the restricted requirements in that policy, an IND is needed for all clinical examinations of items that are subject to section 505 of the FD&C Act.
Cannabinol for Beginners
Based on offered evidence, FDA has actually ended that this is not the instance for THC or CBD. FDA is not familiar with any proof that would certainly cast doubt on its current final Going Here thoughts that THC and also CBD products are left out from the dietary supplement interpretation under area 201(ff)( 3 )(B) of the FD&C Act.
Components that are originated from parts of the marijuana plant that do not consist of THC or CBD may fall outside the extent of this exclusion, as well as as a result may be able to be marketed as nutritional supplements. Nevertheless, all products marketed as nutritional supplements need to abide by all appropriate legislations as well as guidelines regulating dietary supplement products.
355], or a medication for which substantial clinical investigations have been instituted and for which the existence of such investigations has been made public. There are exceptions, including when the medicine was marketed in food prior to the medication was accepted or prior to the considerable professional investigations entailing the drug had been set up or, in the instance of animal feed, that the medicine is a new pet medication approved for usage in feed as well as utilized according to the accepted labeling.
Our Cannabinol Ideas
FDA has actually therefore concluded that it is a restricted act to present or supply for intro right into interstate commerce any kind of food (including any animal food or feed) to which THC or CBD has actually been included. FDA is not knowledgeable about any proof that would cast doubt on these verdicts - cannabinol. Interested events may offer the agency with any kind of evidence that they believe has bearing on this issue.
Active ingredients that are acquired from parts of the cannabis plant that do not include THC or Check This Out CBD could drop outside the extent of 301(ll), and therefore may be able to be included to food. As talked about in Concern # 12, certain hemp seed active ingredients can be legally marketed in human food.
How Cannabinol can Save You Time, Stress, and Money.
For instance, by law, any type of compound intentionally included in food is a preservative, and also consequently based on premarket testimonial and approval by FDA, unless the substance is normally recognized as safe (GRAS) by qualified experts under the conditions of its intended use, or using the compound is otherwise excepted from the interpretation of a food additive (areas 201(s) and also 409 of the FD&C Act [ 21 U.S.C.
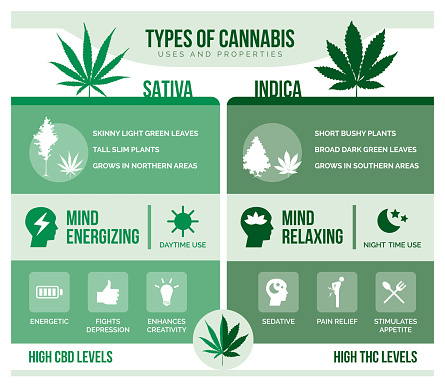
Apart from the three hemp seed active ingredients mentioned in Inquiry # 12, nothing else cannabis or cannabis-derived ingredients have been the subject of a preservative petition, an evaluated GRAS notice, or have otherwise been accepted for use in food by FDA. Food business that want to utilize cannabis or cannabis-derived active ingredients in their foods go through the pertinent laws as well as regulations that control all food, including those that connect to the artificial additive and GRAS procedures.
These GRAS notifications connected just to making use of these active ingredients in human food. To day, FDA has actually not obtained any kind of GRAS notifications for the use of hemp-derived components in animal food (see Question # 25). Hemp seeds are the seeds of the Cannabis sativa plant. The seeds of the plant do not normally have THC or CBD.